An important physical property of a liquid is its boiling point, which is the transformation point of a compound from its liquid phase to its gas phase. The definition of boiling point is the temperature at which the vapor pressure of a liquid equals the external (atmospheric) pressure above that liquid.
When considering the boiling points for a homologous series of molecules, molecular weight is an important factor. Table 4.1 lists the boiling points for several common homologous series. As you look at the table, notice that within a series the boiling points quickly in-crease. For each series, the boiling point shows a fairly regular increase of 20-30oC with each additional CH2 group in the chain. Also, the boiling points vary drastically based on the functional group that the molecule contains.
The molecules in the liquid phase are still close enough to each other that their physical interactions are similar to the physical interactions that occur between the same molecules in their solid phase. Although the interactions in the liquid phase are far more random, and thus generally weaker, than in the solid phase, they still occur and are important. In the gas phase, the molecules are so far apart that the intermolecular interactions are much weaker and are generally of little importance. Except for highly polar substances, such as carboxylic acids, there are essentially no interactions between most molecules in the gas phase.
The interactions between molecules are the result of attractions between those molecules. These attractions fit into three different categories: van der Waals forces, dipolar attractions, and hydrogen bonding. The energies associated with these interactions are small compared to those associated with chemical bonds, but for a collection of molecules, they are significant.
When a pair of molecules approach each other, the nonbonded electrons on one molecule tend to attract the partially positive atoms on the other molecule. These attractive forces increase until they reach a maximum at intermolecular distances between 200 and 400 pm. At distances closer than this, the molecules tend to repel one another because their electrons repel one another. The actual distances at which the molecules begin to repel one another is the sum of the van der Waals radii of the two groups. The average distance between molecules in the liquid phase is in the range of 200-400 pm.
A complementary polarization occurs when a temporary dipole in one molecule induces a similar dipole in another molecule.
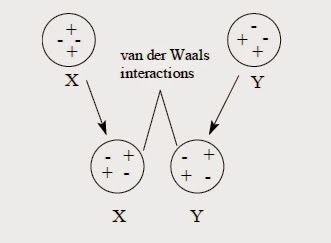
To understand what happens with these attractive forces, consider two nonpolar molecules, X and Y. Keep in mind that the distribution of electrons in these molecules is continually fluctuating. As the two molecules approach each other, they experience a mutual attraction, and any polarization in molecule X induces a complementary polarization in molecule Y. Chemists call this at-traction van der Waals forces or London dispersion forces.
The interactions between molecules are the result of attractions between those molecules. These attractions fit into three different categories: van der Waals forces, dipolar attractions, and hydrogen bonding. The energies associated with these interactions are small compared to those associated with chemical bonds, but for a collection of molecules, they are significant.
When a pair of molecules approach each other, the nonbonded electrons on one molecule tend to attract the partially positive atoms on the other molecule. These attractive forces increase until they reach a maximum at intermolecular distances between 200 and 400 pm. At distances closer than this, the molecules tend to repel one another because their electrons repel one another. The actual distances at which the molecules begin to repel one another is the sum of the van der Waals radii of the two groups. The average distance between molecules in the liquid phase is in the range of 200-400 pm.
A complementary polarization occurs when a temporary dipole in one molecule induces a similar dipole in another molecule.
To understand what happens with these attractive forces, consider two nonpolar molecules, X and Y. Keep in mind that the distribution of electrons in these molecules is continually fluctuating. As the two molecules approach each other, they experience a mutual attraction, and any polarization in molecule X induces a complementary polarization in molecule Y. Chemists call this at-traction van der Waals forces or London dispersion forces.
van der Waals forces, which are sometimes called induced polarizations or induced dipoles, are only temporary and are constantly changing because the electron distribution within each molecule rapidly fluctuates. When the polarization in one molecule changes, it influences a neighboring molecule, which in turn influences another neighboring molecule. The net effect is that all neighboring molecules are attracted to each other. The magnitude of van der Waals forces is based on the number of electrons in the molecules and how many of those electrons participate in these induced dipole-dipole interactions.
For a low polarity liquid to boil, it must overcome the van der Waals forces. The major factor in the magnitude of these forces is the shape of the molecule. Highly branched molecules have a more spherical shape and smaller van der Waals attractions. Unbranched molecules have more surface area that can be involved in intermolecular interactions and higher van der Waals attractions because they can pack closer. You can see this effect in the boiling points of the following three isomers: pentane, 2-methylbutane, and 2,2-dimethylpropane.
For a low polarity liquid to boil, it must overcome the van der Waals forces. The major factor in the magnitude of these forces is the shape of the molecule. Highly branched molecules have a more spherical shape and smaller van der Waals attractions. Unbranched molecules have more surface area that can be involved in intermolecular interactions and higher van der Waals attractions because they can pack closer. You can see this effect in the boiling points of the following three isomers: pentane, 2-methylbutane, and 2,2-dimethylpropane.
Individual van der Waals forces are very weak. However, a typical molecule can participate in so many polarization interactions that the van der Waals forces are among the most important of the intermolecular forces in the liquid phase. They are the only forces possible for nonpolar molecules.
The second category of attractions that occurs between molecules is dipolar attractions. Molecules with permanent dipoles have dipolar attractions because of the charge polarization in their bonds. The interactions between molecules with permanent dipoles are similar to the van der Waals interactions between molecules with induced dipoles. The only difference is that the dipoles are permanent. Methyl fluoride illustrates the interaction of molecules with a permanent dipole. Methyl fluoride has a very polar C—F bond with a partial positive charge on the carbon and a partial negative charge on the fluorine atom:
In the liquid form, many other molecules of methyl fluoride surround each individual molecule of methyl fluoride. All these molecules tend to line up with the negative end of one dipole associated with the positive end of another:
As with van der Waals forces, molecules with dipole attractions require energy to overcome these forces. The dipolar forces raise the boiling point of methyl fluoride above that of a comparable compound without electronegative substituents. For example, methyl fluoride and ethane, have similar molecular weights, but methyl fluoride boils at –78oC whereas ethane boils at –89oC.
The third category of interactions that affects the boiling point is hydrogen bonding. Hydrogen bonding is a type of weak bonding interaction that involves a hydrogen bond donor and a hydrogen bond acceptor. A hydrogen bond donor is a molecule containing a hydrogen attached to an electronegative atom. The most common electronegative atoms in organic molecules are oxygen and nitrogen. A hydrogen bond acceptor is a molecule containing an atom with a nonbonding pair of electrons. The best hydrogen bond acceptors in organic molecules are also oxygen and nitrogen.
The third category of interactions that affects the boiling point is hydrogen bonding. Hydrogen bonding is a type of weak bonding interaction that involves a hydrogen bond donor and a hydrogen bond acceptor. A hydrogen bond donor is a molecule containing a hydrogen attached to an electronegative atom. The most common electronegative atoms in organic molecules are oxygen and nitrogen. A hydrogen bond acceptor is a molecule containing an atom with a nonbonding pair of electrons. The best hydrogen bond acceptors in organic molecules are also oxygen and nitrogen.
The strongest hydrogen bonds are with the O—H group. Weaker hydrogen bonds form with N—H bonds. Much weaker still are the hydrogen bonds formed with S—H and P—H bonds. The strength of an individual hydrogen bond is roughly 5 kcal/mole, much smaller than the typical covalent bond strengths of 80-100 kcal/mole. Hydrogen bonds are stronger than dipolar interactions, which are about 1-2 kcal/mole.
Of the three types of attractive forces, hydrogen bonding is the strongest. Hydrogen bonding substantially raises the boiling points of the compounds in which it occurs. For example, the isomeric compounds dimethyl ether and ethanol have widely different boiling points due to hydrogen bonding in ethanol.
Of the three types of attractive forces, hydrogen bonding is the strongest. Hydrogen bonding substantially raises the boiling points of the compounds in which it occurs. For example, the isomeric compounds dimethyl ether and ethanol have widely different boiling points due to hydrogen bonding in ethanol.
Dimethyl ether has no hydrogen atoms attached to the oxygen, so no hydrogen bonding is possible. However, ethanol has a hydrogen attached to the oxygen, so hydrogen bonding occurs.
Chemists consider hydrogen bonding a very weak or partial bonding between an oxygen of one molecule and a hydrogen of another. This bonding causes aggregations, or groupings, of molecules much like those resulting from dipolar attractions. However, these molecular aggregations possess much more stability than those resulting from dipolar interactions.
Chemists consider hydrogen bonding a very weak or partial bonding between an oxygen of one molecule and a hydrogen of another. This bonding causes aggregations, or groupings, of molecules much like those resulting from dipolar attractions. However, these molecular aggregations possess much more stability than those resulting from dipolar interactions.
Similar aggregations of molecules occur with amines. However, the boiling point differences of isomeric amines are less dramatic than for isomeric compounds of oxygen. For example, 3-methyl-1-butanamine boils at 95-96oC whereas N,N-dimethylpropanamine boils at 65oC.
The smaller difference in boiling points suggests that hydrogen bonds with N—H bonds are weaker than hydrogen bonds with O—H bonds. The N—H bonds are less polar because nitrogen has a lower electronegativity than oxygen. The hydrogen bonds are weaker because the hydrogen end of the dipole in the N—H bond is less positive than that in the O—H bond.
The previous discussion considers intermolecular hydrogen bonding. An additional factor comes into effect when two functional groups in one molecule participate in hydrogen bonding. The resultant intramolecular hydrogen bond is much more important than an intermolecular hydrogen bond in determining the properties of the molecule. For example, 2-nitrophenol has a much lower boiling point than either of its isomers, 3-nitrophenol or 4-nitrophenol.
2-Nitrophenol forms an intramolecular hydrogen bond between the hydrogen of the O—H bond and one of the oxygens in the NO2 group. This intramolecular hydrogen bond prevents an intermolecular hydrogen bond from forming. Thus, boiling requires much less energy for the 2-nitrophenol isomer than for the 4-nitrophenol isomer because there are no strong intermolecular forces to overcome in going from the liquid phase to the gas phase.
2-Nitrophenol represents a category of compounds in which intramolecular hydrogen bonding forms either a stable five- or six-membered ring. Section 3.5, page 000 discusses the relative stability of rings of various sizes. Because of this stability, they form more readily than rings of any other size. Any process that can result in a five- or six-membered ring is favored over one yielding another ring size. Expect to see this pattern repeatedly in your study of organic chemistry.










No comments:
Post a Comment